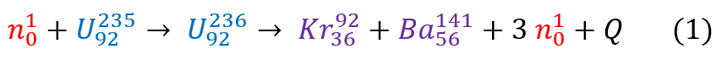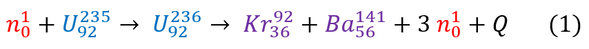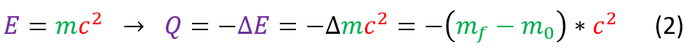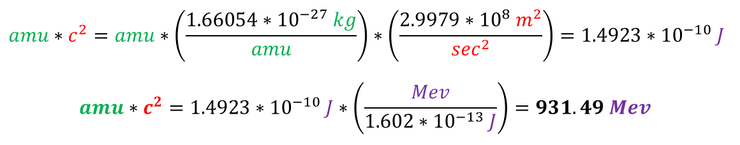Mind Network - Samuel Solomon
Introduction to Fission
Energy Extraction
As we begin our journey into Nuclear Fusion, I think it is important to address one of the most fundamentally confusing questions everyone begins the field asking: how do we extract energy from atomic nuclei. For years scientists have tackled and discussed this problem and have come up with two polar opposite solutions: fusion and fission.
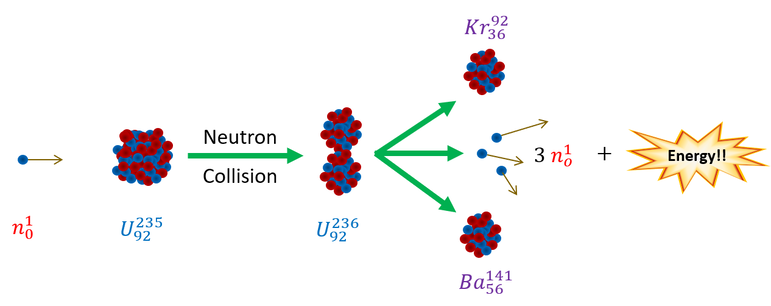
First let us define fission. Fission is the process of splitting a nucleus apart in the hopes of gaining more energy out than that which was supplied to break it. This actually works very well; just think about a bomb. When broken, unstable heavy nuclei can release massive amounts of energy as they break apart. We can pictorially represent this below:
First let us define fission. Fission is the process of splitting a nucleus apart in the hopes of gaining more energy out than that which was supplied to break it. This actually works very well; just think about a bomb. When broken, unstable heavy nuclei can release massive amounts of energy as they break apart. We can pictorially represent this below:
For Uranium-235 fission, a neutron is accelerated at a relatively stable Uranium-235 atom (half life: 703,800,000 years). As it collides (at super high speeds), it fuses into the nucleus to create an unstable Uranium-236 atom which will spontaneously reorganize and fall apart into 2 smaller (and more stable) nuclei: Krypton-92 and Barium -141 as well as 3 neutrons. We can represent this reaction with an easy to read equation below (note: mass number is shown on top ; number of protons is shown on bottom):
We will call the energy released during this reaction the 'Q-value.' For any chemists or biologists reading this page, the Q-value is identical to the enthalpy of a reaction, except it has the opposite sign. To be clear:
* Endothermic reactions = more energy in than out -> have positive enthalpies and negative Q-values.
* Exothermic reactions = release more energy than inputted -> have negative enthalpies and positive Q-values.
The discrepancy in sign is because chemists and biologists care more about how much energy they need to supply a system and nuclear physicists care more about how much energy they can get out of a system. Either way, in the end, it is really just notation (the magnitude is the same).
Without all the engineering dilemmas, this is really the basic (grossly simplified) overview of fission. A few curious thinkers may start to wonder though: where exactly is this released energy coming from. To answer this question, let us first think about all the ways we can get energy into or out of our system (not completely exhaustive, but the most common ways):
1. External supply of internal energy: change the gravitational potential (height) of the nuclei
2. Adding heat to the system: externally raise the temperature, and hence kinetic energy, of the nuclei
3. Increasing the chemical potential of the system: adding more molecules, which inherently carry more energy
4. Work done on or by the system: As the system expands, the atoms push particles away (using some of its energy)
5. Mass converting to energy: All mass can be converted directly into energy (energy-mass balance)
In the fission reaction above (equation 1), we have a closed system and are not adding any extra internal energy, heat, or molecules into our system. Our system is also not expanding or compressing. The only other way there could possibly be any energy gain is if some of the initial mass was converted into energy (take a second to think about this, as it is a super important concept going forwards).
This concept may seem a bit tricky at first, especially when one looks back at our first equation:
* Endothermic reactions = more energy in than out -> have positive enthalpies and negative Q-values.
* Exothermic reactions = release more energy than inputted -> have negative enthalpies and positive Q-values.
The discrepancy in sign is because chemists and biologists care more about how much energy they need to supply a system and nuclear physicists care more about how much energy they can get out of a system. Either way, in the end, it is really just notation (the magnitude is the same).
Without all the engineering dilemmas, this is really the basic (grossly simplified) overview of fission. A few curious thinkers may start to wonder though: where exactly is this released energy coming from. To answer this question, let us first think about all the ways we can get energy into or out of our system (not completely exhaustive, but the most common ways):
1. External supply of internal energy: change the gravitational potential (height) of the nuclei
2. Adding heat to the system: externally raise the temperature, and hence kinetic energy, of the nuclei
3. Increasing the chemical potential of the system: adding more molecules, which inherently carry more energy
4. Work done on or by the system: As the system expands, the atoms push particles away (using some of its energy)
5. Mass converting to energy: All mass can be converted directly into energy (energy-mass balance)
In the fission reaction above (equation 1), we have a closed system and are not adding any extra internal energy, heat, or molecules into our system. Our system is also not expanding or compressing. The only other way there could possibly be any energy gain is if some of the initial mass was converted into energy (take a second to think about this, as it is a super important concept going forwards).
This concept may seem a bit tricky at first, especially when one looks back at our first equation:
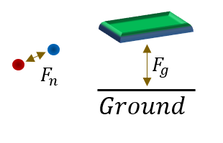
One can immediately notice that, in this reaction diagram, the number of nucleons (protons and neutrons) are conserved. I can also tell you that the number of electrons are conserved as well. So how is there mass being turned into energy if the number of particles remain the same? The answer lies in how the nucleus is bound together.
The nucleus is just a conglomeration of protons (depicted red) and neutrons (depicted blue), and one might remember that while neutrons are neutral, PROTONS ARE NOT! In fact protons are positive and repel each other. So why does the nucleus (with all its protons) stay together? It stays together because it is bound together by a short range strong nuclear force (labeled F_n above) that exists between nucleons. This holds enormous amounts of potential energy, just like gravity (labeled F_g above). Think about a book held above the ground. It may not be moving, or seem like it has any energy, but once you let go you can tell that it did indeed have some energy.
By fissing a nucleus, we are reorganizing these strong nuclear bonds (by adding and removing some). If the net product of this reorganization is more stable, requires less energy to bind the nucleons together, than the excess energy can be released into the environment (usually in the form of kinetic energy of the products), which we can extract as electricity later on.
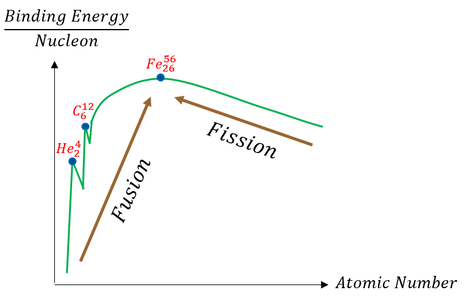
We can get a sense of this binding energy difference between nuclei in the graph below:
By fissing a nucleus, we are reorganizing these strong nuclear bonds (by adding and removing some). If the net product of this reorganization is more stable, requires less energy to bind the nucleons together, than the excess energy can be released into the environment (usually in the form of kinetic energy of the products), which we can extract as electricity later on.
We can get a sense of this binding energy difference between nuclei in the graph below:
Note: the graph is roughly drawn (not to scale). As one can see, the atom with the most binding energy, most stability, is Iron-56. Breaking nuclei apart and fusing nuclei together to Iron-56 would release energy. Be aware that some graphs may invert this image. Just depends on how you define energy stability (negative or positive).
So to circle back, where does this energy come from? The answer lies in the mass. While the nucleons are conserved, we find that some of their mass (that was used to bind the nucleons together) is missing. We can experimentally observe this if we weigh out the particles before and after the reaction. By some extremely small amount, the products are indeed a little bit lighter than the initial reactants. Taking this mass, and Einstein's famous mass-energy equation, we can find that:
So to circle back, where does this energy come from? The answer lies in the mass. While the nucleons are conserved, we find that some of their mass (that was used to bind the nucleons together) is missing. We can experimentally observe this if we weigh out the particles before and after the reaction. By some extremely small amount, the products are indeed a little bit lighter than the initial reactants. Taking this mass, and Einstein's famous mass-energy equation, we can find that:
Note: the energy E normally refers to the energy being added to a system, but Q is the energy released (hence the negative sign). We can therefore convert mass (normally given in amu) into energy (normally shown in Mev for fission and fusion reactions). The conversion factor used is shown below:
Now that we have discussed what a fission reaction is, let us quickly point out all of its problems (this is a fusion course, not fission, after all).
1. Fission is extremely hard to control: an uncontrolled fission reaction is called a bomb
2. Because of this lack of control, it is extremely dangerous: many have died working on fission (see Demon Core)
3. Waste products for fission are radioactive and cannot be disposed of until > 100 years: not environmentally friendly
4. The ease of turning this research into weapons is troubling ... no explanation necessary
5. Unfortunately, regardless of its potential (if used right), public opinion isn't in favor of fission (see above)
It is unfortunate given all the potential for fission energy (this is clean energy; no carbon/ fossil fuels needed), but the fact of the matter is that scientists still have to worry about public opinion. Fission reactors work extremely well when smart people run them. They have build in safety parameters like negative temperature coefficients, moderators, and control rods to SCRAM.
Luckily, for nuclear scientists, there is another option for nuclear energy, which is the exact reverse process of fission, called Fusion. Throughout this course, we will solely focus on discussing where fusion is today and the physics that makes it happen.
1. Fission is extremely hard to control: an uncontrolled fission reaction is called a bomb
2. Because of this lack of control, it is extremely dangerous: many have died working on fission (see Demon Core)
3. Waste products for fission are radioactive and cannot be disposed of until > 100 years: not environmentally friendly
4. The ease of turning this research into weapons is troubling ... no explanation necessary
5. Unfortunately, regardless of its potential (if used right), public opinion isn't in favor of fission (see above)
It is unfortunate given all the potential for fission energy (this is clean energy; no carbon/ fossil fuels needed), but the fact of the matter is that scientists still have to worry about public opinion. Fission reactors work extremely well when smart people run them. They have build in safety parameters like negative temperature coefficients, moderators, and control rods to SCRAM.
Luckily, for nuclear scientists, there is another option for nuclear energy, which is the exact reverse process of fission, called Fusion. Throughout this course, we will solely focus on discussing where fusion is today and the physics that makes it happen.
|
|
|