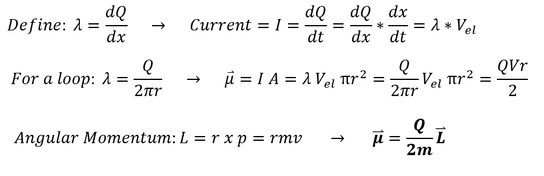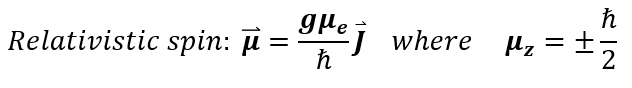Mind Network - Samuel Solomon
Angular Momentum
Experiments
|
|
|
So far we have discussed the linear momentum, position, and energy of a quantum particle. The next quantity we will discuss is the angular momentum. Currently, we believe that every particle has two types of angular momentum: extrinsic and intrinsic. Extrinsic angular momentum is what we classically think of angular momentum: a particle spinning with some momentum around some radius. Intrinsic angular momentum is a bit more tricky to define, but in quantum mechanics it is what is commonly called 'spin.' The exact way in which we think of each of these quantities will be discussed at further length in 'Quantum Mechanics II,' but for now let us start building up our angular momentum principles. While theoretical work is how we advance and pursue previously un-thinkable applications, it is always best to ground oneself in the physical experiments. Let us consider the two famous experiments that laid the foundation for how physicists understand quantum angular momentum:
Einstein-De Hass Experiment
The experiment is performed as follows:
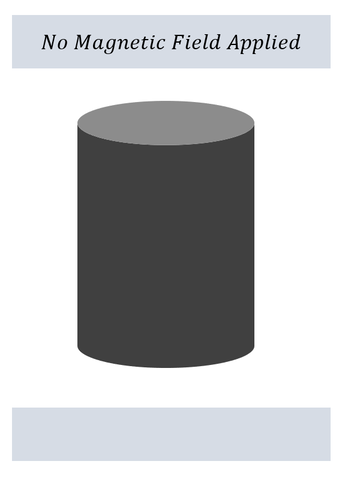
On the left: An iron rod is placed between two magnets without a magnetic field
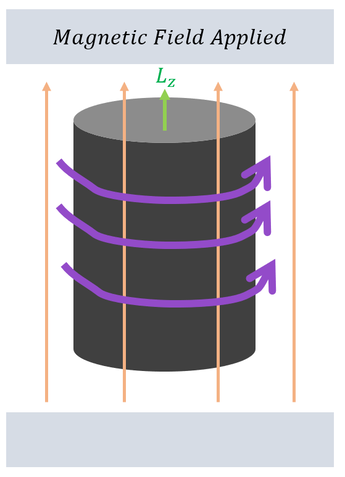
On the right: An iron rod is placed between two magnets with a magnetic field applied.
On the left: An iron rod is placed between two magnets without a magnetic field
On the right: An iron rod is placed between two magnets with a magnetic field applied.
The experimental observations can be summarized below:
On the left: The iron rod does not move. Nothing out of the ordinary is observed
On the right: The iron rod begins to rotate around the axis of the magnetic field with some angular momentum.
In order to analyze this experiment we first must ask ourselves: what does applying a magnetic field do to a system?
On the left: The iron rod does not move. Nothing out of the ordinary is observed
On the right: The iron rod begins to rotate around the axis of the magnetic field with some angular momentum.
In order to analyze this experiment we first must ask ourselves: what does applying a magnetic field do to a system?
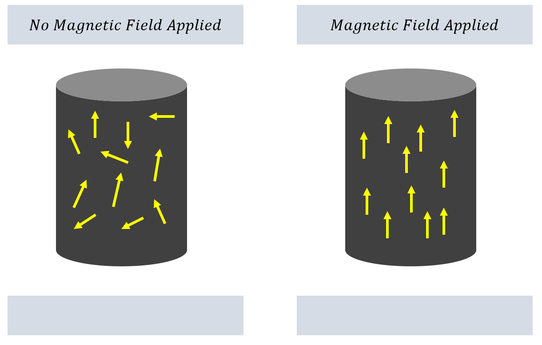
Before quantum mechanics, physicists already knew that applying a magnetic field to a dipole aligns the dipole in the direction of the magnetic field. A dipole is just a separation of two charges; hence, all atoms exhibit dipoles. Normally, in metals, these dipoles all randomly align and cancel each other out. Once a magnetic field is applied to the metal, we know that one difference (at least) between the two states is the alignment of the dipoles in the metal.
To recap: Applying a magnetic field aligns all the dipoles in the metal; applying a magnetic field also causes the metal to spin. While at this stage we have too little information to really connect these two concepts, we can at least start our angular momentum analysis with a hypothesis in mind: There exists some angular momentum aspect of a dipole; the angular momentum has some relationship (parallel relationship) with the magnetic field.
To recap: Applying a magnetic field aligns all the dipoles in the metal; applying a magnetic field also causes the metal to spin. While at this stage we have too little information to really connect these two concepts, we can at least start our angular momentum analysis with a hypothesis in mind: There exists some angular momentum aspect of a dipole; the angular momentum has some relationship (parallel relationship) with the magnetic field.
Stern-Gerlach Experiment
The experiment is performed as follows:
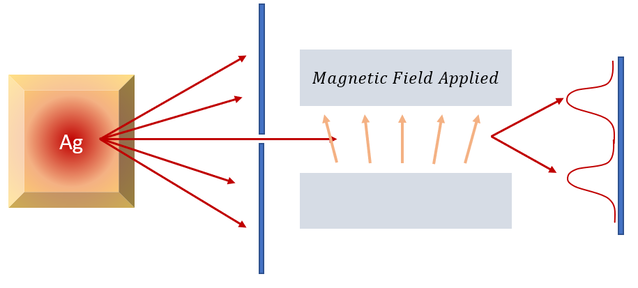
Pure silver atoms were heated up in an oven until they vaporized. While the particles flew in all directions, a portion of the particles were focused through a small hole (particles entered going only in one direction). Once through, the particles were subjected to a magnetic field along the an axis (a magnetic field along the Z-direction is commonly used). The magnetic field was slightly varying along the axis.
Pure silver atoms were heated up in an oven until they vaporized. While the particles flew in all directions, a portion of the particles were focused through a small hole (particles entered going only in one direction). Once through, the particles were subjected to a magnetic field along the an axis (a magnetic field along the Z-direction is commonly used). The magnetic field was slightly varying along the axis.
The experimental observations can be summarized below:
1. With an applied magnetic field, the particles are deflected into two general regions
2. Without a magnetic field, the particles are not deflected and make one general peak in the center
Previously, in electromagnetism, physicists understood how dipoles behaved in changing magnetic field. To understand this experiment, let us go over what was know about a particle in a changing magnetic field.
1. With an applied magnetic field, the particles are deflected into two general regions
2. Without a magnetic field, the particles are not deflected and make one general peak in the center
Previously, in electromagnetism, physicists understood how dipoles behaved in changing magnetic field. To understand this experiment, let us go over what was know about a particle in a changing magnetic field.
In order to make sure everyone is following, let us review some key steps below:
We defined some 'lambda' to be the linear charge density (where Q = charge). Starting from current (charge/second),
we found a relationship between the current and this new value 'lambda'
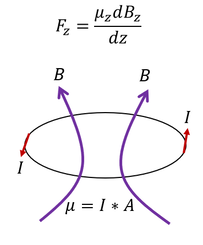
For a magnetic field in a loop, the dipole μ is defined as the current * area. For a uniform charge, the charge density is
just the total charge 'Q' over the total distance (the circumference).
Angular momentum is just L = r * m * v for 'r' perpendicular to 'v.' We can plug this relationship into the μ (dipole)
equation we found earlier.
From the last bold equation, we find that the dipole μ is directly proportional to the angular momentum L (both are vectors and 'Q/2m' is a scalar). This makes sense since the Einstein-De Hass experiment predicted some angular momentum and dipole proportionality (now we mathematically start to see it). In reality, atoms are not electrons on a wire, but we are still working our way up. We are becoming more firm, experimentally and mathematically, in the idea that the dipole and the angular momentum relate.
Now it is time to go back to the experiment at hand. The force that a magnetic field exerts on a dipole (see the equation above the ring on the left) is directly proportional to the amount the dipole aligns with the changing magnetic field; hence, for the same dipole and magnetic field, different dipole orientations in space will give different overlaps and different forces exerted. If the particles feel different forces, then they would be deflected in all different directions.
But we only see 2 different peaks (the spread of the peak represent some Gaussian distribution of the initial velocities and experimental errors. Same spreading is seen for no magnetic field). Given that the magnetic field in the experiment is the same and the particles are the same, the only difference can be their dipole. If we saw a continuous peak, we would expect a continuous spectrum of dipole orientations and deflections, but we do not. There are only 2 different peaks for 2 different dipole orientations.
The dipole is also proportional to the angular momentum. If we have a quantization of dipole directions, we also have a quantization of angular momentum (like what Bohr predicted).
We defined some 'lambda' to be the linear charge density (where Q = charge). Starting from current (charge/second),
we found a relationship between the current and this new value 'lambda'
For a magnetic field in a loop, the dipole μ is defined as the current * area. For a uniform charge, the charge density is
just the total charge 'Q' over the total distance (the circumference).
Angular momentum is just L = r * m * v for 'r' perpendicular to 'v.' We can plug this relationship into the μ (dipole)
equation we found earlier.
From the last bold equation, we find that the dipole μ is directly proportional to the angular momentum L (both are vectors and 'Q/2m' is a scalar). This makes sense since the Einstein-De Hass experiment predicted some angular momentum and dipole proportionality (now we mathematically start to see it). In reality, atoms are not electrons on a wire, but we are still working our way up. We are becoming more firm, experimentally and mathematically, in the idea that the dipole and the angular momentum relate.
Now it is time to go back to the experiment at hand. The force that a magnetic field exerts on a dipole (see the equation above the ring on the left) is directly proportional to the amount the dipole aligns with the changing magnetic field; hence, for the same dipole and magnetic field, different dipole orientations in space will give different overlaps and different forces exerted. If the particles feel different forces, then they would be deflected in all different directions.
But we only see 2 different peaks (the spread of the peak represent some Gaussian distribution of the initial velocities and experimental errors. Same spreading is seen for no magnetic field). Given that the magnetic field in the experiment is the same and the particles are the same, the only difference can be their dipole. If we saw a continuous peak, we would expect a continuous spectrum of dipole orientations and deflections, but we do not. There are only 2 different peaks for 2 different dipole orientations.
The dipole is also proportional to the angular momentum. If we have a quantization of dipole directions, we also have a quantization of angular momentum (like what Bohr predicted).
Pullback to What we Know
Let us review what these experiments taught us so far:
1. The dipole of an atom is somehow related (proportionally) to the angular momentum
2. There are only 2 dipole values
3. The dipole and angular momentum are quantized.
Unproven yet, but how we actually understand these experiments today:
Einstein-De Hass: atoms have two types of angular momentum: intrinsic spin and extrinsic rotation. Together they make up the total angular momentum and, like in classical mechanics, is conserved. Without a magnetic field, the system has a fixed (and conserved) amount of total angular momentum: in part from the intrinsic and in part from extrinsic angular momentum. When the dipoles align, the intrinsic angular momentum changes, resulting in the total angular momentum changing (but it cant because it is conserved: constant in time). In order to counter this addition of intrinsic angular momentum, the system adds in an extrinsic angular momentum to cancel it out (and keep the same total angular momentum). This extrinsic angular momentum is what we see when we see the rod spinning.
Stern-Gerlach: We only have two different types of intrinsic angular momentum: spin up and spin down. Every particle, electron and proton and neutron, have a spin up or spin down value (although the electron spins tend to be the one that mathematically matters). When the particles enter the changing magnetic field, around half are spin up and half are spin down (since the particles were not created in a way of favoring one spin over the other). The spin up is deflected one way and the spin down is deflected the other way. Interestingly enough, one of the only reasons the silver exhibited this two deflection property (unbeknownst to the experimenters) was because silver has exactly one unpaired electron. All the paired electrons, when shot into the changing magnetic field, feel a force up and a force down (but they cancel each other out. They are not magnetically active). However, the one unpaired electron in silver will feel the force depending on its spin and it is this spin that deflects the silver atoms. If we had two unpaired electrons, we would see more than two peaks.
Experimentally, the value of the two types of dipoles have been measured and found to be 'h_bar/2' and ' - h_bar/2.' The true equation, which we will not get into in this course, for the dipole can be sen below:
1. The dipole of an atom is somehow related (proportionally) to the angular momentum
2. There are only 2 dipole values
3. The dipole and angular momentum are quantized.
Unproven yet, but how we actually understand these experiments today:
Einstein-De Hass: atoms have two types of angular momentum: intrinsic spin and extrinsic rotation. Together they make up the total angular momentum and, like in classical mechanics, is conserved. Without a magnetic field, the system has a fixed (and conserved) amount of total angular momentum: in part from the intrinsic and in part from extrinsic angular momentum. When the dipoles align, the intrinsic angular momentum changes, resulting in the total angular momentum changing (but it cant because it is conserved: constant in time). In order to counter this addition of intrinsic angular momentum, the system adds in an extrinsic angular momentum to cancel it out (and keep the same total angular momentum). This extrinsic angular momentum is what we see when we see the rod spinning.
Stern-Gerlach: We only have two different types of intrinsic angular momentum: spin up and spin down. Every particle, electron and proton and neutron, have a spin up or spin down value (although the electron spins tend to be the one that mathematically matters). When the particles enter the changing magnetic field, around half are spin up and half are spin down (since the particles were not created in a way of favoring one spin over the other). The spin up is deflected one way and the spin down is deflected the other way. Interestingly enough, one of the only reasons the silver exhibited this two deflection property (unbeknownst to the experimenters) was because silver has exactly one unpaired electron. All the paired electrons, when shot into the changing magnetic field, feel a force up and a force down (but they cancel each other out. They are not magnetically active). However, the one unpaired electron in silver will feel the force depending on its spin and it is this spin that deflects the silver atoms. If we had two unpaired electrons, we would see more than two peaks.
Experimentally, the value of the two types of dipoles have been measured and found to be 'h_bar/2' and ' - h_bar/2.' The true equation, which we will not get into in this course, for the dipole can be sen below:
Where J = the total angular momentum (intrinsic spin + extrinsic rotation); g = the gyromagnetic ratio = g.00236930419922(2) for an electron (unknown for a muon); μ_e is the 'Bohr magneton' which is a contant = 9.274009994(57) × 10−24 Joules / Tesla.
|
|
|