Mind Network - Samuel Solomon
Choosing Fusion Reactants
Okay, so lets do some fusion. Lets throw some atoms at each other and BAM! collect the energy. We are done!
Unfortunately, life isn't this simple ... as previously stated there is a high coulomb scattering probability and we need to supply TONS of energy to fuse two atoms. If we supply all this energy to get this reaction going, we better be making more energy out than in (I mean theoretically, we haven't even started discussing the engineering challenges associated).
In short, we need to decide which atoms we are fusing to maximize the energy out before we start theorizing crazy fusion designs. So theorists did some number crunching (using today's technology standards) with 3 main constraints:
1. Small coulomb scatter cross section: less nuclear charge = less nuclear repulsion (atoms are ions in plasma)
2. High fusion cross section: eliminate all reactants with an extremely small fusion cross section
3. High energy released: eliminate all reactants with small energy output (maximize Q-value)
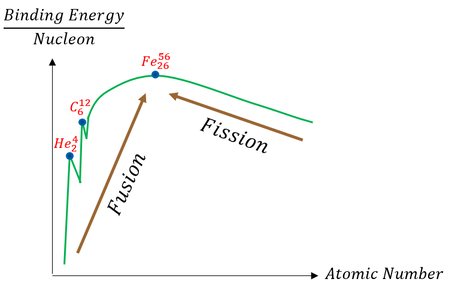
To answer these questions, let us first look back at our relative graph of fusion binding energy:
Unfortunately, life isn't this simple ... as previously stated there is a high coulomb scattering probability and we need to supply TONS of energy to fuse two atoms. If we supply all this energy to get this reaction going, we better be making more energy out than in (I mean theoretically, we haven't even started discussing the engineering challenges associated).
In short, we need to decide which atoms we are fusing to maximize the energy out before we start theorizing crazy fusion designs. So theorists did some number crunching (using today's technology standards) with 3 main constraints:
1. Small coulomb scatter cross section: less nuclear charge = less nuclear repulsion (atoms are ions in plasma)
2. High fusion cross section: eliminate all reactants with an extremely small fusion cross section
3. High energy released: eliminate all reactants with small energy output (maximize Q-value)
To answer these questions, let us first look back at our relative graph of fusion binding energy:
Just to be transparent, I made the above graph (relatively matching real data out there). Qualitatively one can see though that we can maximize energy output (reactant-product energy differences; Q-value) by sticking with low Z-values. Furthermore, since we are inputting TONS of energy to make 2 atoms fuse (so much we are in a plasma state), all atoms are ions. Ions repel each other. Lower nuclear charge (lower Z-value) would mean a lower coulomb barrier (less repulsion).
While calculations for many reactants have been performed, we can qualitatively appreciate that intuitively lower Z-values are going to be easier to fuse. Specifically, the calculations say that we must have a Z(atom 1) * Z(atom 2) > 7.
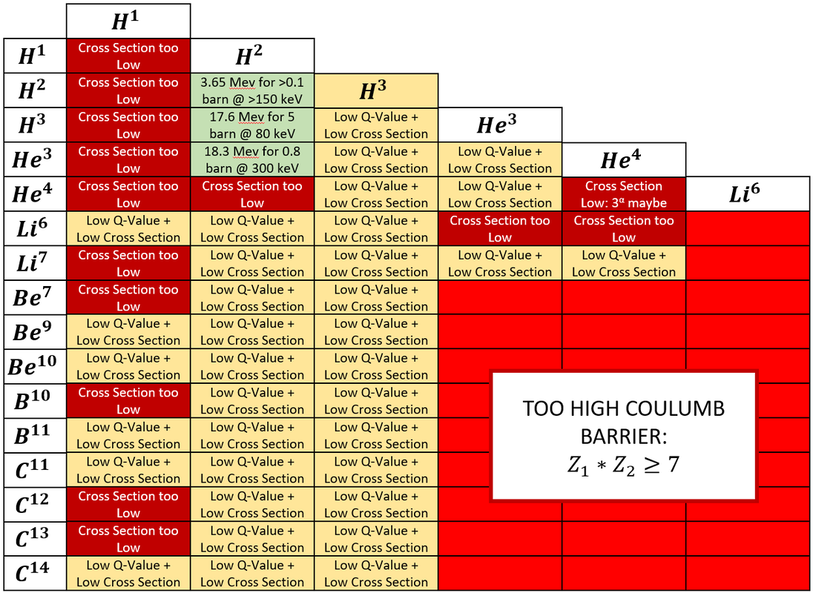
As we continue on, please note that I will be neglecting atoms with an extremely small half life (unstable, t_1/2 < 1 min) as well as 3-body fusion (2 atoms colliding is hard enough). We will now constrict our search for nuclear reactants to the pairs below:
While calculations for many reactants have been performed, we can qualitatively appreciate that intuitively lower Z-values are going to be easier to fuse. Specifically, the calculations say that we must have a Z(atom 1) * Z(atom 2) > 7.
As we continue on, please note that I will be neglecting atoms with an extremely small half life (unstable, t_1/2 < 1 min) as well as 3-body fusion (2 atoms colliding is hard enough). We will now constrict our search for nuclear reactants to the pairs below:
After crossing out low fusion cross sections, high coulomb scattering cross sections, and low energy outputs (low Q-value reactions), we are left with 3 possible fusion reactants. ONLY 3!! ... Yikes ...
To be clear, the yellow above doesn't mean impossible, it just means less likely theoretically. Both shades of red means impossible. The 3 green reactions should theoretically work better than the yellow (and they are taking years of hard work with little success, so that should say how unlikely the yellow reactions are).
So now it is time to weed down our three contestants (plus one I threw in for fun). We can take a closer look at their specifics below:
To be clear, the yellow above doesn't mean impossible, it just means less likely theoretically. Both shades of red means impossible. The 3 green reactions should theoretically work better than the yellow (and they are taking years of hard work with little success, so that should say how unlikely the yellow reactions are).
So now it is time to weed down our three contestants (plus one I threw in for fun). We can take a closer look at their specifics below:
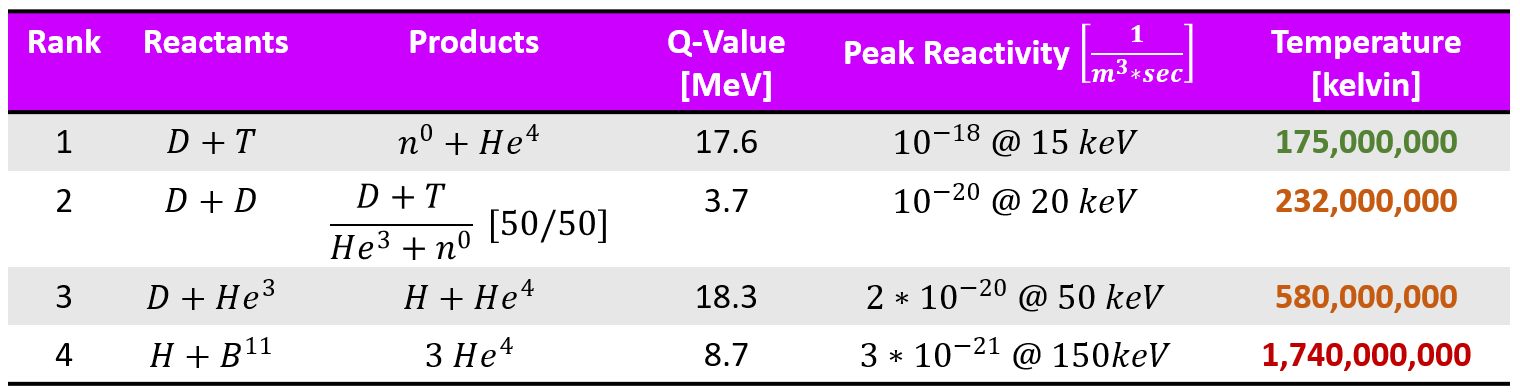
Unpacking the table above, while the ranking is done holistically, one can easily see that the most important factor in a successful fusion is the energy required to make the atoms fuse. This is because sustaining up to 100's of millions of Kelvin (room temperature is about 300 Kelvin) in a plasma is HARD and requires TONS of input energy. For a machine that is suppose to net produce energy, we need to limit this input barrier.
The second most important quality is the Q-value and the Reactivity. We need a species that will fuse easily as well as produce more energy than we supplied (which as we said, is a lot). We can summarize the top 3 contenders below.
D-T Reaction
Just by looking at the above numbers, one can easily see why most scientists believe that the best fusion reactants are Deuterium (D) and Tritium (T). The pair has the highest reactivity and lowest temperature to react. It does not have the highest Q-value but it is comparable to the highest. It is the most studied in fusion research. Most of the 17.6 MeV Q-value can be recovered (we will discuss how this occurs later). The biggest drawback, however, is that there is no natural source of tritium on earth. The half life of Tritium is 12.33 years.
D-D Reaction
The D-D reaction has two branches, producing He-3 + neutron as well as tritium + hydrogen. The biggest pro for D-D fusion is that Deuterium is a very common and inexpensive material (can be extracted from the ocean).
D-He-3 Reaction
Major drawback to this reaction is that it requires He-3 as a fuel. There are no natural sources of He-3 on earth (though it is stable unlike tritium). The biggest pro for this reaction is that it not only has the highest viable Q-value, but the reaction produces NO NEUTRONS (I know, this is sacrilegious for many nuclear physicists). No neutrons though means less radiation damage and material activation. However, this also leads to a problem as much of the energy extraction comes from the neutron produced. Without a neutron, one would need to get the energy out of the charged particle (the proton specifically in this case). There is some technical hurdles involved there.
Overall, a majority of scientists have leaned towards Deuterium and Tritium as the main fusion reactants, and hence this course will specifically focus on those fusion systems. Nonetheless, it is important to realize/ take note of the other possibilities out there (you never know when they may become more viable).
The second most important quality is the Q-value and the Reactivity. We need a species that will fuse easily as well as produce more energy than we supplied (which as we said, is a lot). We can summarize the top 3 contenders below.
D-T Reaction
Just by looking at the above numbers, one can easily see why most scientists believe that the best fusion reactants are Deuterium (D) and Tritium (T). The pair has the highest reactivity and lowest temperature to react. It does not have the highest Q-value but it is comparable to the highest. It is the most studied in fusion research. Most of the 17.6 MeV Q-value can be recovered (we will discuss how this occurs later). The biggest drawback, however, is that there is no natural source of tritium on earth. The half life of Tritium is 12.33 years.
D-D Reaction
The D-D reaction has two branches, producing He-3 + neutron as well as tritium + hydrogen. The biggest pro for D-D fusion is that Deuterium is a very common and inexpensive material (can be extracted from the ocean).
D-He-3 Reaction
Major drawback to this reaction is that it requires He-3 as a fuel. There are no natural sources of He-3 on earth (though it is stable unlike tritium). The biggest pro for this reaction is that it not only has the highest viable Q-value, but the reaction produces NO NEUTRONS (I know, this is sacrilegious for many nuclear physicists). No neutrons though means less radiation damage and material activation. However, this also leads to a problem as much of the energy extraction comes from the neutron produced. Without a neutron, one would need to get the energy out of the charged particle (the proton specifically in this case). There is some technical hurdles involved there.
Overall, a majority of scientists have leaned towards Deuterium and Tritium as the main fusion reactants, and hence this course will specifically focus on those fusion systems. Nonetheless, it is important to realize/ take note of the other possibilities out there (you never know when they may become more viable).
|
|
|